Understanding atomic spectra is fundamental to both physics and chemistry, particularly in the context of the MDCAT syllabus. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms, which arise from the transitions of electrons between energy levels. This chapter delves into the intricate world of atomic spectra, exploring its definition, the distinction between atomic absorption and emission spectra, and the various types that exist. By grasping these concepts, students can better appreciate the underlying principles that govern atomic behavior and the interactions of light with matter. The knowledge of atomic spectra not only aids in answering theoretical questions but also equips students with the skills to tackle multiple-choice questions (MCQs) effectively. As we navigate through this topic, we will highlight key aspects, such as the significance of spectral lines, the role of the Bohr model in explaining atomic spectra, and the practical applications of these phenomena in fields like spectroscopy and quantum mechanics. Prepare to unlock the mysteries of atomic spectra and enhance your understanding of this captivating subject!
Atomic spectra refer to the distinct patterns of light emitted or absorbed by atoms when electrons transition between energy levels. These spectra are crucial in both physics and chemistry for understanding atomic structure and the behavior of matter under electromagnetic radiation. There are two primary types of atomic spectra: emission spectra, which display bright lines against a dark background, and absorption spectra, characterized by dark lines in a continuous spectrum. The study of atomic spectra allows scientists to identify elements and their concentrations in various substances, making it a fundamental concept in spectroscopy. Understanding atomic spectra is essential for grasping concepts like energy quantization, the Bohr model of the atom, and the nature of electromagnetic radiation. This knowledge is particularly relevant for students preparing for exams like the MDCAT, where questions on atomic spectra frequently appear.

Atomic spectra are fascinating phenomena that reveal the secrets of the atomic world! They refer to the spectrum of electromagnetic radiation emitted or absorbed by an atom. But what does that really mean? Let's break it down!
The study of atomic spectra is essential in various scientific fields, including:
Each element emits or absorbs light at specific wavelengths, making it possible to identify them based on their atomic spectra. This uniqueness is crucial for:
Understanding atomic spectra also involves recognizing the different types:
By exploring these questions, we can deepen our understanding of atomic spectra and their significance in science!
Did you know that atomic spectra can also help astronomers determine the chemical composition of stars? By analyzing the light emitted from these celestial bodies, scientists can uncover the mysteries of the universe!
In summary, atomic spectra are not just a scientific curiosity; they are a vital tool in understanding the building blocks of matter and the universe itself. Whether you're studying physics, chemistry, or astronomy, grasping the concept of atomic spectra is essential for unlocking the secrets of the atomic world! 🌟
| Aspect | Description | Examples | Applications |
|---|---|---|---|
| Definition | The spectrum of electromagnetic radiation emitted or absorbed by an atom during electron transitions. | Emission and Absorption Spectra | Used in spectroscopy for identifying elements. |
| Types | Includes continuous spectrum, line spectrum, and band spectrum. | Continuous spectrum (rainbow), Line spectrum (hydrogen spectrum) | Applied in astrophysics and chemical analysis. |
| Importance | Provides insights into the electronic structure of atoms and chemical bonds. | Unique spectral fingerprints for each element. | Used in analytical chemistry and astrophysics. |
| Applications | Identifying elements and compounds, determining composition of stars and galaxies. | Spectroscopy in labs and telescopes. | Essential for chemical analysis and research. |
Understanding the different types of atomic spectra is crucial for grasping the behavior of atoms and their interactions with light. Let's dive into the two primary types: emission spectra and absorption spectra.
What is it?
The emission spectrum is produced when an atom emits light. This occurs during the transition of electrons from a higher energy level to a lower one.
How does it look?
The emitted light appears as bright lines against a dark background. Each line corresponds to a specific wavelength, which indicates the energy difference between the levels.
Key Points:
What is it?
An absorption spectrum occurs when an atom absorbs light, causing electrons to jump from a lower energy level to a higher one.
How does it look?
This spectrum appears as dark lines superimposed on a continuous spectrum. The dark lines represent the specific wavelengths of light that have been absorbed by the atom.
Key Points:
Both emission and absorption spectra are essential for understanding the electronic structure of atoms. They have numerous applications, including:
- Identifying elements in various samples.
- Studying chemical reactions and their mechanisms.
- Exploring astrophysical phenomena by analyzing light from stars.
By grasping the concepts of atomic spectra, you can unlock a deeper understanding of the universe around you! 🌌✨

| Type of Spectrum | Definition | Appearance | Energy Transition | Applications |
|---|---|---|---|---|
| Emission Spectrum | Produced when an atom emits light as electrons transition from a higher to a lower energy level. | Bright lines against a dark background. | Electrons move from higher to lower energy levels, releasing energy as light. | Used in identifying elements and studying chemical reactions. |
| Absorption Spectrum | Occurs when an atom absorbs light, causing electrons to move from a lower to a higher energy level. | Dark lines superimposed on a continuous spectrum. | Electrons move from lower to higher energy levels, absorbing energy from light. | Used in spectroscopy to analyze the composition of substances. |
Niels Bohr, a Danish physicist, introduced a groundbreaking model of the atom in the early 20th century that transformed our understanding of atomic structure. His model is particularly significant in explaining atomic spectra, which are the unique patterns of light emitted or absorbed by atoms. Let’s dive deeper into the key aspects of Bohr's atomic model!
Fixed Orbits: Bohr proposed that electrons move in fixed paths or orbits around the nucleus. Each orbit corresponds to a specific energy level, which means that:
Energy Levels: The energy associated with each orbit is quantized. This means:
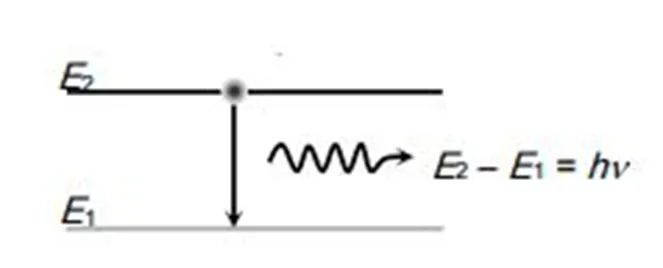
Photon Emission and Absorption: The energy of the emitted or absorbed photon corresponds to the difference in energy between the two orbits:
Bohr's model successfully explains the spectral lines observed in hydrogen, which was a monumental achievement in physics. Here are some important points regarding atomic spectra:
While Bohr's atomic model was revolutionary, it does have its limitations:
- Multi-Electron Atoms: The model primarily applies to hydrogen and struggles to accurately describe more complex atoms with multiple electrons.
- Electron-Electron Interactions: It does not account for the interactions between electrons, which are significant in multi-electron systems.
For those interested in diving deeper into the topic, here are some avenues to explore:
- Difference Between Atomic Absorption and Emission Spectra: Understanding how these two processes differ can enhance your grasp of atomic behavior.
- Atomic Spectra in Physics: This topic is crucial for various applications in physics, including spectroscopy and quantum mechanics.
- Practice Questions: Engage with atomic spectra questions and MCQs to test your knowledge and reinforce your understanding.
In summary, Bohr's atomic model not only laid the groundwork for modern quantum mechanics but also provided a clear framework for understanding atomic spectra. By grasping these concepts, you can appreciate the intricate dance of electrons around the nucleus and the light they emit or absorb! 🌟

| Type | Description | Example | Applications |
|---|---|---|---|
| Emission Spectrum | Produced when electrons fall from a higher energy level to a lower one, releasing energy as light. | Hydrogen emission spectrum showing distinct lines at specific wavelengths. | Used in spectroscopy to identify elements in stars. |
| Absorption Spectrum | Created when electrons absorb energy and move to a higher energy level, resulting in dark lines on a continuous spectrum. | Hydrogen absorption spectrum showing dark lines superimposed on a continuous spectrum. | Used in determining the composition of gases in the atmosphere. |
| Continuous Spectrum | A complete range of wavelengths without gaps, showing all colors blending into one another. | Rainbow produced by sunlight passing through a prism. | Used in understanding the properties of light and its interaction with matter. |
The hydrogen atom, the simplest atom in the universe, has fascinating properties that can be described using mathematical expressions. In this section, we will explore the key equations that define the energy levels, radii of orbits, and the wavelengths of emitted radiation in hydrogen. 🌌
In Bohr's model, the energy levels of the hydrogen atom are quantized, meaning that electrons can only occupy specific energy levels. The formulas that describe these levels are crucial for understanding atomic spectra.
The radius of the nth orbit can be calculated using the formula:
r_n = n²h² / (4π²kZe²)
This formula shows how the radius increases with the square of the principal quantum number, leading to larger orbits for higher energy levels. 📏
The energy of the electron in the nth orbit is given by:
E_n = - (2π²mk²e⁴) / (n²h²)
This expression reveals that the energy is negative, indicating that the electron is bound to the nucleus. The more negative the energy, the more stable the electron's position in the atom. ⚡
When electrons transition between energy levels, they emit or absorb radiation. The wavelength of this radiation can be calculated using the Rydberg formula:
1/λ = R_H(1/n_f² - 1/n_i²)
This formula is essential for understanding the different types of atomic spectra produced during these transitions. 🌈
The study of atomic spectra is vital in physics and chemistry. Here are some related concepts to explore:
By grasping these mathematical expressions and their implications, we can better understand the behavior of the hydrogen atom and its role in the broader context of atomic spectra. 🌟

| Principal Quantum Number (n) | Radius of nth Orbit (r_n) | Energy of nth Orbit (E_n) | Wavelength of Emitted Radiation (1/λ) |
|---|---|---|---|
| 1 | 0.53 Å | -13.6 eV | R_H(1/1² - 1/n_i²) |
| 2 | 2.12 Å | -3.4 eV | R_H(1/2² - 1/n_i²) |
| 3 | 4.75 Å | -1.51 eV | R_H(1/3² - 1/n_i²) |
| 4 | 8.60 Å | -0.85 eV | R_H(1/4² - 1/n_i²) |
Understanding Spectral Lines and Series 🌈
Spectral lines are fascinating features observed in the spectrum of light emitted or absorbed by atoms. These distinct lines correspond to specific wavelengths of light, which are produced when electrons transition between different energy levels within an atom. This phenomenon is crucial for understanding atomic spectra, which reveal a lot about the structure and behavior of atoms.
The hydrogen atom, one of the simplest atoms, exhibits several spectral series based on the final energy level of the electron. Here are the main series:
Lyman Series:
Balmer Series:
Paschen Series:
Bracket and Pfund Series:
By diving into the world of spectral lines and series, you not only learn about the fundamental aspects of atomic structure but also gain insights into the universe's workings. Keep exploring the wonders of atomic spectra! 🌟

| Series | Final Energy Level (n) | Type of Light | Wavelength Range |
|---|---|---|---|
| Lyman Series | 1 | Ultraviolet | < 400 nm |
| Balmer Series | 2 | Visible | 400 nm - 700 nm |
| Paschen Series | 3 | Infrared | > 700 nm |
| Bracket Series | 4 | Infrared | Varies |
| Pfund Series | 5 | Infrared | Varies |
When diving into the world of atoms, two key concepts come to the forefront: excitation potential and ionization potential. These terms are essential for grasping how atoms interact with energy, and they play a crucial role in the study of atomic spectra. Let’s break them down!
Understanding excitation and ionization potentials is crucial for explaining various phenomena, including:
By grasping these concepts, you will not only enhance your understanding of atomic behavior but also prepare yourself for more complex topics in physics and chemistry. Keep exploring the fascinating world of atomic spectra! 🌌✨

| Property | Excitation Potential | Ionization Potential |
|---|---|---|
| Definition | Energy required to move an electron from ground state to an excited state within the same atom. | Energy required to completely remove an electron from an atom, resulting in the formation of a cation. |
| Energy Level | Raises the electron to a higher energy level without removing it from the atom. | Completely removes the electron from the atom. |
| Magnitude | Always lower than ionization potential. | Always greater than excitation potential. |
| Relation to Atomic Spectra | Relevant for understanding atomic emission spectra as it involves energy transitions within the atom. | Relevant for understanding the formation of cations and the overall stability of the atom. |
| Applications | Used to explain phenomena such as atomic emission spectra. | Used to explain the stability of atoms and their reactivity. |
The absorption and emission of radiation are key processes in the realm of atomic physics. Understanding these phenomena is crucial for grasping how atoms interact with light and energy. Let’s dive deeper into these fascinating concepts!
When an atom absorbs energy, its electrons get excited and jump to higher energy levels. This process can be summarized as follows:
Conversely, when electrons return to their original, lower energy levels, they release energy in the form of light. Here’s how it works:
Both absorption and emission processes are governed by the principles of quantum mechanics. This branch of physics helps explain:
To clarify further, here are the main differences between atomic absorption and emission spectra:
Process:
Spectrum Appearance:
Applications:
Understanding the different types of atomic spectra can enhance your knowledge:
By exploring these topics, you can gain a deeper understanding of atomic spectra and their significance in physics. Whether you're preparing for exams or just curious about the universe, mastering these concepts will serve you well!

| Property | Atomic Absorption Spectrum | Atomic Emission Spectrum |
|---|---|---|
| Definition | Spectrum produced when electrons absorb energy and move to higher energy levels. | Spectrum produced when electrons release energy and fall to lower energy levels. |
| Appearance | Characterized by dark lines superimposed on a continuous spectrum. | Characterized by bright lines on a dark background. |
| Energy Transition | Involves absorption of energy by electrons. | Involves emission of energy by electrons. |
| Applications | Used in techniques such as atomic absorption spectroscopy for element analysis. | Used in techniques such as emission spectroscopy for identifying elements. |
| Examples | Commonly seen in elements like sodium and potassium in flame tests. | Commonly seen in neon signs and spectral lamps. |
The Spectral Series of the Hydrogen Atom 🌌
The spectral series of the hydrogen atom is a fascinating aspect of atomic physics, showcasing the unique behavior of electrons as they transition between different energy levels. These transitions result in the emission or absorption of light at specific wavelengths, creating a series of spectral lines that are characteristic of hydrogen. Let's dive deeper into the most notable series:
These spectral series can be mathematically described by the Rydberg formula, which provides a way to calculate the wavelengths of the emitted or absorbed light. This formula highlights the quantized nature of energy levels in hydrogen, illustrating how electrons occupy specific orbits around the nucleus.
Understanding the spectral series of hydrogen is essential for grasping the fundamentals of atomic spectra. It provides insights into:
- The structure of atoms.
- The behavior of electrons.
- The nature of light and its interaction with matter.
By exploring the spectral series of the hydrogen atom, we gain a deeper appreciation for the intricate dance of electrons and the light they produce, which is fundamental to both physics and astronomy. 🌟
| Series | Transition Level | Type of Light | Wavelength Range |
|---|---|---|---|
| Lyman Series | n=1 | Ultraviolet | < 400 nm |
| Balmer Series | n=2 | Visible | 400-700 nm |
| Paschen Series | n=3 | Infrared | 700 nm - 1 mm |
| Bracket Series | n=4 | Infrared | 1 mm - 10 mm |
| Pfund Series | n=5 | Far Infrared | > 10 mm |
Applications of Atomic Spectra 🌌
Atomic spectra play a crucial role in various scientific fields, providing insights that enhance our understanding of the universe and the materials within it. Let's explore some of the most significant applications of atomic spectra:
The applications of atomic spectra are vast and varied, impacting fields from astrophysics to medicine. Understanding these applications not only highlights the significance of atomic spectra in scientific research but also emphasizes their practical uses in everyday life.
By exploring the difference between atomic absorption and emission spectra, one can gain deeper insights into how these techniques are applied in various analyses. For those curious about the atomic spectra definition or looking for atomic spectra questions, resources are plentiful. Additionally, understanding the types of atomic spectra can enhance knowledge in physics and chemistry, making it easier to tackle atomic spectra MCQs in academic settings.
| Field | Application | Description |
|---|---|---|
| Astrophysics | Spectral Analysis | Identifying the composition of stars and galaxies through spectral analysis. |
| Chemical Analysis | Atomic Absorption Spectroscopy (AAS) | Determining the concentration of elements in samples. |
| Medical Diagnostics | Spectroscopy in Diagnostics | Analyzing biological samples using various spectroscopy techniques. |
| Material Science | Spectral Characteristics | Understanding the properties of materials through their spectral characteristics. |
In summary, atomic spectra are not just a fascinating topic; they are a gateway to understanding the very fabric of our universe! Let's dive deeper into the key insights and implications of this essential subject.
Mastering the concepts of atomic spectra is crucial for students aiming for success in:
- Exams: Understanding these principles can significantly boost your performance in physics and chemistry.
- Careers: Fields such as research, healthcare, and engineering rely heavily on the knowledge of atomic spectra.
Understanding atomic spectra opens up a world of possibilities in science and technology. By grasping these concepts, you not only prepare for academic challenges but also equip yourself for a future in scientific innovation!
With these insights, you're now better prepared to tackle questions related to the difference between atomic absorption and emission spectra, explore atomic spectra definitions, and engage with atomic spectra questions. Remember, the journey into the world of atomic spectra is just beginning!
| Concept | Description | Key Points |
|---|---|---|
| Atomic Spectra | The spectrum of electromagnetic radiation emitted or absorbed by atoms. | - Provides insights into atomic structure and behavior. |
| Emission Spectra | Produced when electrons fall to lower energy levels, emitting light. | - Characterized by bright lines on a dark background. |
| Absorption Spectra | Formed when electrons absorb energy and move to higher energy levels. | - Characterized by dark lines on a bright background. |
| Bohr's Atomic Model | Describes quantized energy levels of electrons in an atom. | - Key to understanding atomic spectra. |
| Spectral Series | Groups of spectral lines corresponding to transitions between energy levels. | - Includes Lyman, Balmer, and Paschen series. |
| Applications | Used in fields like astrophysics, chemistry, and medicine. | - Essential for identifying elements and compounds. |
In conclusion, the study of atomic spectra is essential for understanding the behavior of atoms as they interact with electromagnetic radiation. This chapter has explored the various types of spectra, including emission and absorption spectra, and their significance in identifying elements. The insights gained from concepts such as Bohr's atomic model and the mathematical expressions related to atomic energy levels enhance our comprehension of atomic structure. By grasping the differences between atomic absorption and emission spectra, students can better appreciate the practical applications of atomic spectra in fields like spectroscopy and quantum mechanics. As you prepare for your MDCAT journey, remember that a thorough understanding of atomic spectra will not only aid in your exams but also lay a solid foundation for future studies in physics and chemistry.
A: Atomic spectra refer to the characteristic wavelengths of electromagnetic radiation emitted or absorbed by atoms. When electrons in an atom transition between energy levels, they emit or absorb light at specific wavelengths, resulting in a spectrum that can be analyzed to identify elements.
A: There are two main types of atomic spectra: emission spectra and absorption spectra. Emission spectra consist of bright lines on a dark background, produced when electrons fall from higher to lower energy levels. Absorption spectra show dark lines on a bright background, indicating wavelengths absorbed by electrons moving to higher energy levels.
A: The key difference is that atomic absorption spectra occur when atoms absorb specific wavelengths of light, resulting in dark lines in the spectrum, while atomic emission spectra occur when atoms emit light at specific wavelengths, resulting in bright lines. Both spectra provide valuable information about the energy levels of electrons in atoms.
A: The Bohr model explains atomic spectra by proposing that electrons orbit the nucleus in fixed energy levels. When an electron transitions between these levels, it emits or absorbs energy in the form of photons, corresponding to specific wavelengths of light, which form the atomic spectrum.
A: Atomic spectra are significant in physics as they provide insights into the electronic structure of atoms, allowing scientists to identify elements and their concentrations in various samples. They are also crucial in fields like astrophysics, where they help analyze the composition of stars and galaxies.
A: Common questions related to atomic spectra include definitions, types, differences between absorption and emission spectra, the role of the Bohr model, and applications in identifying elements in various contexts.
A: Typical MCQs on atomic spectra may cover topics such as the types of spectra, the significance of spectral lines, the Bohr model's predictions, and the applications of atomic spectra in real-world scenarios, such as spectroscopy and astrophysics.