Alkyl halides, also known as haloalkanes, are a fascinating class of organic compounds characterized by the presence of one or more halogen atoms (such as fluorine, chlorine, bromine, or iodine) bonded to an alkyl group. These compounds play a crucial role in both synthetic organic chemistry and various industrial applications. Understanding the structure, reactivity, and classification of alkyl halides is essential for students preparing for the MDCAT, as these concepts form the foundation for more complex organic reactions and mechanisms.
In this blog post, we will delve into the various aspects of alkyl halides, including their general formula, classification based on the number of carbon atoms bonded to the halogen, and the key reactions they undergo. We will explore the mechanisms of nucleophilic substitution and elimination reactions, which are pivotal in the transformation of alkyl halides into other functional groups, such as alcohols and aldehydes. Additionally, we will discuss the reactivity patterns of alkyl halides, including their behavior in dehydrohalogenation and reduction reactions, and how solvents like DMSO can influence these processes.
By the end of this post, readers will have a comprehensive understanding of alkyl halides, equipping them with the knowledge necessary to tackle related questions in the MDCAT and beyond. Whether you are curious about the alkyl halide formula, the preparation methods, or the intricacies of their reactivity, this article will serve as a valuable resource for your studies.
Alkyl halides, also known as haloalkanes, are organic compounds in which one or more hydrogen atoms in an alkane are replaced by halogen atoms, such as fluorine, chlorine, bromine, or iodine. The general formula for alkyl halides is CnH2n+1X, where X represents the halogen atom. Alkyl halides are classified based on the number of alkyl groups attached to the carbon atom bonded to the halogen: primary (1°), secondary (2°), and tertiary (3°) alkyl halides. Their reactivity is influenced by factors such as bond energy and polarity, with iodine being the most reactive halogen and fluorine the least. Alkyl halides undergo various reactions, including nucleophilic substitution and elimination reactions, making them important intermediates in organic synthesis. Understanding their structure, reactivity, and preparation methods is essential for mastering organic chemistry concepts.

Alkyl halides, commonly referred to as haloalkanes, are fascinating organic compounds that play a significant role in various chemical reactions. These compounds are formed when one or more hydrogen atoms in an alkane are replaced by halogen atoms such as fluorine, chlorine, bromine, or iodine. Understanding the classification of alkyl halides is crucial for predicting their reactivity and the mechanisms of their reactions. Let’s dive into the different types of alkyl halides! 🔍
Alkyl halides can be classified based on the number of carbon atoms attached to the carbon atom that is bonded to the halogen. Here’s a breakdown:
Primary Alkyl Halide 🥇
 (Image for visualization)
(Image for visualization)Secondary Alkyl Halide 🥈
 (Image for visualization)
(Image for visualization)Tertiary Alkyl Halide 🥉
 (Image for visualization)
(Image for visualization)Understanding these classifications is essential for several reasons:
- Predicting Reactivity: Different types of alkyl halides exhibit varying reactivity patterns. For instance, tertiary alkyl halides are generally more reactive than primary ones due to steric hindrance.
- Mechanisms of Reactions: The classification helps in predicting the mechanisms of reactions such as dehydrohalogenation and reduction of alkyl halides.
- Applications in Synthesis: Alkyl halides are often used as intermediates in organic synthesis, making their classification vital for chemists.
By grasping the classification of alkyl halides, you can enhance your understanding of organic chemistry and its applications! Happy learning! 🎉

| Type of Alkyl Halide | Description | Example |
|---|---|---|
| Primary Alkyl Halide | The carbon attached to the halogen is bonded to only one other carbon atom. | Bromoethane (C2H5Br) |
| Secondary Alkyl Halide | The carbon bonded to the halogen is attached to two other carbon atoms. | 2-Bromopropane (C3H7Br) |
| Tertiary Alkyl Halide | The carbon carrying the halogen is bonded to three other carbon atoms. | Tert-butyl bromide (C4H9Br) |
The reactivity of alkyl halides is a fascinating topic in organic chemistry, primarily influenced by two key factors: bond energy and bond polarity. Let’s dive deeper into these concepts to understand why some alkyl halides are more reactive than others.
The bond energy refers to the strength of the carbon-halogen bond. A weaker bond means that it is easier to break, leading to higher reactivity. Here’s the general order of reactivity based on the halogen present:
This order indicates that alkyl iodides are the most reactive due to the weaker C-I bond compared to the C-Br, C-Cl, and C-F bonds. This makes alkyl iodides more favorable in nucleophilic substitution reactions.
In addition to bond energy, the polarity of the carbon-halogen bond also plays a significant role in reactivity. The general order of bond polarity is:
Here, the polarity affects how the alkyl halide interacts with nucleophiles. A more polar bond can lead to a stronger interaction with nucleophiles, enhancing reactivity.
If you're curious about the broader implications and applications of alkyl halides, consider exploring these related topics:
Understanding the reactivity of alkyl halides is crucial for mastering organic synthesis and reaction mechanisms. By grasping these fundamental concepts, you can enhance your knowledge and application of organic chemistry! 🌟
| Halogen | Bond Energy (kJ/mol) | Reactivity Order | Bond Polarity |
|---|---|---|---|
| Iodine (I) | ~ 240 | Most Reactive | Lowest Polarity |
| Bromine (Br) | ~ 290 | Moderately Reactive | Low Polarity |
| Chlorine (Cl) | ~ 340 | Less Reactive | Moderate Polarity |
| Fluorine (F) | ~ 430 | Least Reactive | Highest Polarity |
In the fascinating world of organic chemistry, two key players are nucleophiles and electrophiles. These species are essential for understanding various chemical reactions, particularly those involving alkyl halides.
Nucleophiles are species that donate an electron pair to form a chemical bond. This means they are rich in electrons and are often negatively charged or neutral with a lone pair of electrons. Here are some common examples of nucleophiles:
These nucleophiles play a crucial role in attacking electrophiles during chemical reactions.
On the flip side, electrophiles are species that accept an electron pair. They are typically electron-deficient, making them attractive targets for nucleophiles. A prime example of an electrophile is alkyl halides.
The interaction between nucleophiles and electrophiles is fundamental in several important reactions, including:
By grasping the roles of nucleophiles and electrophiles, along with the behavior of alkyl halides, you can unlock the secrets of many organic reactions. Whether you're exploring the general formula of alkyl halide or learning about the transformation of alkyl halide to aldehyde, these concepts are foundational to your chemistry journey! 🌈

| Species | Type | Role in Reaction | Examples |
|---|---|---|---|
| Alkyl Halides | Electrophile | Act as electron acceptors due to the polar C-X bond | Bromoethane, Chloroethane |
| Hydroxide Ion (OH-) | Nucleophile | Donates electron pair to form a bond | Sodium hydroxide (NaOH) |
| Alkoxide Ion (RO-) | Nucleophile | Donates electron pair to form a bond | Sodium ethoxide (NaOEt) |
| Amines (RNH2) | Nucleophile | Donates electron pair to form a bond | Methylamine, Ethylamine |
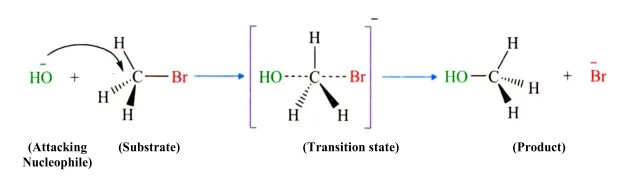
The SN2 reaction is a fascinating and essential mechanism in organic chemistry, particularly when dealing with alkyl halides. This one-step process involves a nucleophile attacking an electrophilic carbon atom, leading to a simultaneous bond formation and bond breaking. Let's dive deeper into the key characteristics of this reaction!
Molecularity:
Kinetics:
Stereochemistry:
Primary Alkyl Halides:
Secondary and Tertiary Alkyl Halides:
If you're interested in learning more about alkyl halides and their reactions, consider exploring the following topics:
By understanding the SN2 reaction and its relationship with alkyl halides, you will gain valuable insights into organic chemistry and its practical applications! 🧪✨

| Characteristic | Description |
|---|---|
| Molecularity | Bimolecular (involves two reactants: alkyl halide and nucleophile) |
| Kinetics | Rate = k [Substrate] [Nucleophile] |
| Stereochemistry | Inversion of configuration at the carbon center (stereospecific) |
| Favorability | Favored by primary alkyl halides due to less steric hindrance |
The SN1 reaction is a fascinating chemical process that occurs in two distinct steps. Understanding this mechanism is crucial for anyone diving into the world of organic chemistry, especially when dealing with alkyl halides. Let's break it down!
Molecularity:
Kinetics:
Stereochemistry:
Understanding the SN1 mechanism not only enhances your grasp of organic reactions but also prepares you for more complex topics in chemistry. Happy studying! 📚✨

| Step | Description | Key Characteristics |
|---|---|---|
| 1. Ionization | The alkyl halide dissociates to form a carbocation and a leaving group. | Rate-determining step; Unimolecular; Rate = k [Substrate] |
| 2. Nucleophilic Attack | The nucleophile attacks the carbocation to form the product. | Can lead to racemization; Favored by tertiary alkyl halides |
Elimination reactions are fascinating processes that involve the removal of atoms or groups from a molecule, leading to the formation of double bonds. When it comes to alkyl halides, these reactions can occur through two primary mechanisms: E1 and E2. Let's dive deeper into each mechanism!
The E1 reaction (unimolecular elimination) is a two-step process that resembles the SN1 mechanism. Here’s how it works:
Formation of Carbocation:
- The first step involves the departure of the leaving group (like a halide ion), resulting in the formation of a carbocation. This intermediate is crucial as it determines the stability of the reaction.
Deprotonation:
- In the second step, a base abstracts a proton from a neighboring carbon, leading to the formation of a double bond.
Key Characteristics of E1 Reactions:
- Favored by Weak Bases: E1 reactions typically occur in the presence of weak bases.
- Solvent Preference: They are most effective in polar protic solvents, which stabilize the carbocation intermediate.
- Tertiary Halides: These reactions are more common with tertiary alkyl halides due to their ability to stabilize the carbocation.
In contrast, the E2 reaction (bimolecular elimination) is a one-step mechanism that is quite efficient. Here’s how it unfolds:
Key Characteristics of E2 Reactions:
- Strong Bases Required: E2 reactions necessitate the use of strong bases, such as sodium hydroxide (NaOH) or potassium tert-butoxide (KOt-Bu).
- Solvent Preference: They are favored in polar aprotic solvents, which do not stabilize the carbocation but rather facilitate the reaction.
- Steric Factors: E2 reactions are more likely to occur with secondary and tertiary alkyl halides due to steric hindrance that discourages substitution reactions.
Both E1 and E2 mechanisms are significantly influenced by the structure of the alkyl halide:
Understanding the differences between E1 and E2 elimination reactions is essential for mastering the chemistry of alkyl halides. Whether you're exploring the dehydrohalogenation of alkyl halides or the reactivity of alkyl halides, knowing these mechanisms will enhance your grasp of organic chemistry concepts.
For more insights, check out related topics like alkyl halide preparation, the general formula of alkyl halide, and the intriguing dmso reaction with alkyl halide! 🌟

| Feature | E1 Reaction | E2 Reaction |
|---|---|---|
| Mechanism Type | Two-step process | One-step process |
| Rate Determining Step | Formation of carbocation | Simultaneous proton abstraction and leaving group departure |
| Base Requirement | Weak bases | Strong bases |
| Solvent Preference | Polar protic solvents | Polar aprotic solvents |
| Structure Preference | Tertiary alkyl halides favored | Tertiary > Secondary > Primary |
| Stereochemistry | Can lead to racemization | Stereochemistry retained or inverted |
When diving into the fascinating world of alkyl halides, it's essential to keep several key points in mind to enhance your understanding and application of these compounds. Here’s a breakdown of the most important concepts:
The reactivity of alkyl halides decreases in the following order:
- R-I (Iodide) >
- R-Br (Bromide) >
- R-Cl (Chloride) >
- R-F (Fluoride)
This order is crucial for predicting how different alkyl halides will behave in reactions.
By keeping these points in mind, you’ll be better equipped to tackle questions about alkyl halides, their properties, and their reactions. Whether you're exploring what are alkyl halides, their general formula, or the dehydrohalogenation of alkyl halides, these foundational concepts will guide your studies and experiments effectively!
| Type of Reaction | Alkyl Halide Preference | Reactivity Order | Key Characteristics |
|---|---|---|---|
| Nucleophilic Substitution (SN2) | Primary Alkyl Halides | R-I > R-Br > R-Cl > R-F | Favors strong nucleophiles and polar aprotic solvents. |
| Nucleophilic Substitution (SN1) | Tertiary Alkyl Halides | R-I > R-Br > R-Cl > R-F | Favors weak nucleophiles and polar protic solvents. |
| Elimination (E1) | Tertiary Alkyl Halides | R-I > R-Br > R-Cl > R-F | Occurs in two steps; influenced by solvent and base strength. |
| Elimination (E2) | Primary Alkyl Halides | R-I > R-Br > R-Cl > R-F | Occurs in one step; requires strong bases. |
Preparing for your exams can be a daunting task, but focusing on important past paper questions can make a significant difference! Below are some key questions related to alkyl halides that you should definitely review. Let’s dive in! 🚀
During an SN2 reaction, what happens to the configuration of the alkyl halide molecule?
- a. Remains the same
- b. Depends upon carbon atom
- c. Gets inverted 🔄
- d. Depends upon electronegativity of halide
When a purely alcoholic solution of sodium/potassium hydroxide and halogen alkene are refluxed, an alkene is formed. What is the mechanism of this reaction?
- a. Dehydration
- b. Elimination ➡️
- c. Debromination
- d. Nucleophilic substitution
The alkaline hydrolysis of bromoethane produces alcohol as the product. What reagent and condition are used in this reaction?
- a. H2O at room temperature
- b. KOH in alcohol
- c. Ethanol, heat
- d. Dilute NaOH(aq), warm 🌡️
In a substitution reaction, what mechanism does a secondary halogenoalkane typically show?
- a. SN1 mechanism 🌀
- b. Both E1 and E2
- c. SN2 mechanism
- d. Both SN1 and SN2
If halogen alkanes are mixed with an excess of ethanoic ammonia and heated under pressure, amines are formed. Which amine is produced in the following reaction?
- CH3-CH2-Br + NH3 -> Amine
- a. CH3-CH2-NH-CH2-CH3
- b. CH3-CH2-CH2-NH2
- c. CH3-CH2-NH2 🧪
- d. H2N-CH2-CH2-NH2
These questions not only help you understand the reactivity of alkyl halides but also prepare you for various mechanisms involved in their reactions. Make sure to review these concepts thoroughly to ace your exams! Good luck! 🍀
| Question Number | Question | Options |
|---|---|---|
| 1 | During SN2 reaction, the configuration of the alkyl halide molecule is: | a. Remains the same |
| b. Depends upon carbon atom | ||
| c. Gets inverted | ||
| d. Depends upon electronegativity of halide | ||
| 2 | When a purely alcoholic solution of sodium/potassium hydroxide and halogen alkene are refluxed, an alkene is formed. What is the mechanism of the reaction? | a. Dehydration |
| b. Elimination | ||
| c. Debromination | ||
| d. Nucleophilic substitution | ||
| 3 | The alkaline hydrolysis of bromoethane gives alcohol as the product. The reagent and the condition used in this reaction may be: | a. H2O at room temperature |
| b. KOH in alcohol | ||
| c. Ethanol, heat | ||
| d. Dilute NaOH(aq), warm | ||
| 4 | In substitution reaction, secondary halogenoalkane shows: | a. SN1 mechanism |
| b. Both E1 and E2 | ||
| c. SN2 mechanism | ||
| d. Both SN1 and SN2 | ||
| 5 | If halogen alkanes are mixed with an excess of ethanoic ammonia and heated under pressure, amines are formed. Which amine is formed in the following reaction? CH3-CH2-Br + NH3 -> Amine | a. CH3-CH2-NH-CH2-CH3 |
| b. CH3-CH2-CH2-NH2 | ||
| c. CH3-CH2-NH2 | ||
| d. H2N-CH2-CH2-NH2 |
In conclusion, alkyl halides play a significant role in organic chemistry due to their unique structure and reactivity. As compounds formed by replacing hydrogen atoms in alkanes with halogen atoms, they exhibit diverse behaviors in nucleophilic substitution and elimination reactions. Understanding the classification of alkyl halides—primary, secondary, and tertiary—along with their reactivity patterns is crucial for mastering their transformations, such as dehydrohalogenation and reduction processes. The mechanisms of these reactions, including SN1 and SN2 pathways, further illustrate the complexity of alkyl halides. By grasping these concepts, students can effectively prepare for the MDCAT and apply their knowledge to real-world chemical scenarios. For further exploration, consider the various applications and reactions involving alkyl halides, such as their conversion to aldehydes and their interactions with solvents like DMSO.
A: Alkyl halides are organic compounds in which one or more hydrogen atoms in an alkane are replaced by halogen atoms (fluorine, chlorine, bromine, or iodine). They are classified based on the carbon atom bonded to the halogen, which can be primary, secondary, or tertiary.
A: The general formula for alkyl halides is CnH2n+1X, where 'X' represents a halogen atom (F, Cl, Br, or I) and 'n' is the number of carbon atoms in the alkyl group.
A: Alkyl halides can be prepared through various methods, including the halogenation of alkanes, the reaction of alcohols with hydrogen halides, and dehydrohalogenation of alkyl halides to form alkenes.
A: The reactivity of alkyl halides is influenced by the type of halogen and the structure of the alkyl group. Generally, the order of reactivity is iodine > bromine > chlorine > fluorine, with primary alkyl halides being more reactive in nucleophilic substitution reactions.
A: Dehydrohalogenation is a reaction where alkyl halides are treated with strong bases to eliminate a hydrogen halide, resulting in the formation of alkenes. This reaction can follow either an E1 or E2 mechanism, depending on the structure of the alkyl halide and the conditions.
A: Alkyl halides can be reduced to form alcohols or alkanes through reactions with reducing agents such as lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4).
A: Yes, alkyl halides can be converted to aldehydes through various methods, including reaction with lithium aluminum hydride (LiAlH4) followed by hydrolysis, or through the use of Grignard reagents followed by oxidation.
A: Dimethyl sulfoxide (DMSO) is often used as a solvent in reactions involving alkyl halides due to its ability to solvate ions and enhance nucleophilic substitution reactions, particularly in SN2 mechanisms.