Carboxylic acids are a fundamental class of organic compounds characterized by the presence of the carboxyl functional group (-COOH). This unique structure not only defines their chemical properties but also plays a crucial role in various biological processes and industrial applications. The general formula for carboxylic acids is RCOOH, where R represents a hydrocarbon chain that can be either aliphatic or aromatic. Understanding the nomenclature, classification, and reactivity of carboxylic acids is essential for students preparing for the MDCAT exam, as these concepts frequently appear in examination questions.
In this chapter, we will delve into the intriguing world of carboxylic acids, exploring their physical properties, preparation methods, and various reactions. From the oxidation of alcohols to the formation of esters, the versatility of carboxylic acids is evident in their ability to participate in numerous organic reactions. Moreover, we will discuss the significance of carboxylic acids in everyday life, from their role in metabolic pathways to their applications in the synthesis of polymers and pharmaceuticals.
As you navigate through this chapter, you will gain a comprehensive understanding of carboxylic acids, equipping you with the knowledge necessary to excel in both your examinations and future studies in organic chemistry. So, let’s embark on this journey to uncover the fascinating aspects of carboxylic acids, their structures, and their myriad of functions in the chemical world.
Carboxylic acids are organic compounds characterized by the presence of a carboxyl functional group (-COOH). This functional group consists of a carbon atom double-bonded to an oxygen atom (carbonyl) and single-bonded to a hydroxyl group (–OH). The general formula for carboxylic acids is R-COOH, where R represents a hydrocarbon chain that can be either aliphatic or aromatic. Carboxylic acids are known for their acidic properties, as they can donate protons (H+) in solution, making them more acidic than alcohols and phenols. Their reactivity includes undergoing esterification, forming esters when reacted with alcohols, and participating in various organic reactions. The nomenclature of carboxylic acids follows specific IUPAC rules, where the suffix '-oic acid' is used to denote their structure. Common examples include acetic acid (ethanoic acid) and citric acid. Understanding carboxylic acids is essential in organic chemistry due to their prevalence in biological systems and industrial applications.

Carboxylic acids are fascinating organic compounds that play a vital role in chemistry, characterized by the presence of the carboxyl functional group (-COOH). Understanding the nomenclature of these acids is essential for anyone diving into the world of organic chemistry. Let's break down the key aspects of naming carboxylic acids!
The International Union of Pure and Applied Chemistry (IUPAC) has established specific rules for naming carboxylic acids. Here’s how it works:
Identify the Longest Carbon Chain:
Replace the 'e':
When it comes to aromatic carboxylic acids, the naming convention is slightly different:
Understanding the nomenclature of carboxylic acids is crucial for:
By mastering the nomenclature of carboxylic acids, you’ll be better equipped to navigate the complexities of organic chemistry and appreciate the role these compounds play in various chemical processes!
Did you know that carboxylic acids are not just limited to laboratory settings? They are found in everyday substances, like vinegar (acetic acid) and citric acid in citrus fruits! 🍋
Now that you have a grasp on the nomenclature of carboxylic acids, you can confidently explore their structures and functions in organic chemistry!

| Type | General Formula | Example | IUPAC Name |
|---|---|---|---|
| Aliphatic Carboxylic Acids | RCOOH | Acetic Acid | Ethanoic Acid |
| Aromatic Carboxylic Acids | C6H5COOH | Benzoic Acid | Benzoic Acid |
Carboxylic acids are fascinating organic compounds that play a crucial role in various chemical processes. They can be classified based on their structure and the number of carboxyl groups they contain. Let’s dive into the primary classifications:
Carboxylic acids can also be categorized based on the nature of their carbon chains:
The carboxylic acid functional group (-COOH) is what defines these compounds. This group is responsible for their acidic properties and reactivity. The general formula for carboxylic acids can be represented as:
Did you know that carboxylic acids can react with sodium carbonate to produce carbon dioxide? This reaction is often used in laboratory settings to demonstrate acid-base reactions.
By understanding the classification of carboxylic acids, we can appreciate their diverse roles in both nature and industry. Whether it’s in food, medicine, or chemical synthesis, these compounds are truly essential!

| Type | Description | Examples |
|---|---|---|
| Monocarboxylic Acids | Contain one carboxyl group | Acetic acid (CH₃COOH), Formic acid (HCOOH) |
| Dicarboxylic Acids | Contain two carboxyl groups | Oxalic acid (C₂H₂O₄), Succinic acid (C₄H₆O₄) |
| Polycarboxylic Acids | Contain three or more carboxyl groups | Citric acid (C₆H₈O₇), Tartaric acid (C₄H₆O₆) |
| Aliphatic Carboxylic Acids | Carboxylic acids with straight or branched carbon chains | Butanoic acid (C₄H₈O₂) |
| Aromatic Carboxylic Acids | Carboxylic acids with a benzene ring | Benzoic acid (C₇H₆O₂) |
Carboxylic acids are fascinating organic compounds characterized by their unique functional group, -COOH. This group not only defines their chemical behavior but also significantly influences their physical properties. Let’s dive into some of the key characteristics of carboxylic acids that make them stand out!
Understanding these physical properties is essential for anyone studying organic chemistry, especially when dealing with the carboxylic acid formula and its applications in various fields. Whether you're exploring the carboxylic acid structure or delving into the conversion of carboxylic acid to alcohol, these properties play a crucial role in their behavior and utility in chemical reactions.

| Property | Description |
|---|---|
| Boiling Points | Carboxylic acids have higher boiling points than alcohols and hydrocarbons of similar molecular weight due to strong hydrogen bonding. |
| Solubility | Lower molecular weight carboxylic acids are soluble in water due to hydrogen bonding, but solubility decreases with increasing carbon chain length. |
| Odor | Many carboxylic acids have distinctive odors; for example, acetic acid has a pungent smell. |
| Color | Most carboxylic acids are colorless, but some can be colored due to impurities or specific structural features. |
Carboxylic acids are an essential class of organic compounds characterized by the presence of the carboxyl group (-COOH). They can be synthesized through various methods, each with its unique approach and application. Below are some key preparation methods that are widely used in organic chemistry:
Understanding these preparation methods is fundamental for anyone studying organic chemistry, especially when dealing with the carboxylic acid functional group. Whether you're looking into the carboxylic acid formula or exploring the conversion of carboxylic acid to alcohol, mastering these techniques will enhance your knowledge and application of organic compounds. 🌟

| Method | Description | Example |
|---|---|---|
| Oxidation of Alcohols | Primary alcohols are oxidized to carboxylic acids using oxidizing agents like potassium dichromate in acidic conditions. | Ethanol (C2H5OH) to Acetic Acid (CH3COOH) |
| Hydrolysis of Nitriles | Nitriles can be hydrolyzed to produce carboxylic acids in the presence of acid or base. | Ethanenitrile (CH3CN) to Acetic Acid (CH3COOH) |
| Carbonation of Grignard Reagents | Grignard reagents react with carbon dioxide to form carboxylic acids upon hydrolysis. | Grignard reagent (RMgX) with CO2 to form RCOOH |
| Decarboxylation of Carboxylic Acids | Certain carboxylic acids can be decarboxylated to yield hydrocarbons. | Sodium acetate (CH3COONa) to Ethane (C2H6) |
Carboxylic acids are fascinating compounds known for their reactivity, primarily due to the presence of the carboxyl group (-COOH). This functional group not only defines their chemical behavior but also plays a crucial role in various reactions. Let's dive deeper into the key reactions involving carboxylic acids:
Carboxylic acids are not just simple compounds; their reactivity opens up a world of possibilities in organic chemistry! Whether you're studying their structure or exploring their reactions, understanding carboxylic acids is key to mastering organic reactions. 🌈

| Reaction Type | Description | Key Features | Example |
|---|---|---|---|
| Acidity | Carboxylic acids can donate protons (H⁺) in aqueous solutions, forming carboxylate ions. | Weak acids, dissociate partially in water, forming carboxylate ions. | Acetic acid (CH₃COOH) dissociates to form acetate ions (CH₃COO⁻). |
| Esterification | Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters. | Reversible reaction, can be driven to completion by removing water. | Acetic acid reacts with ethanol to form ethyl acetate. |
| Formation of Acid Chlorides | Carboxylic acids react with thionyl chloride (SOCl₂) to form acid chlorides. | More reactive derivatives, used in further reactions. | Acetic acid reacts with SOCl₂ to form acetyl chloride. |
| Amide Formation | Carboxylic acids react with amines to form amides, requiring activation of the acid. | Less favorable without activation, involves nucleophilic attack by amines. | Acetic acid reacts with ammonia to form acetamide. |
Esterification: The Art of Creating Esters 🌸
Esterification is a fascinating and essential chemical reaction that plays a pivotal role in organic chemistry. It involves the reaction between carboxylic acids and alcohols to produce esters, which are not only important in chemistry but also in our daily lives, especially in fragrances and flavors.
The general reaction can be summarized as follows:
RCOOH + R'OH ⇌ RCOOR' + H₂O
Esters are known for their pleasant odors and are widely used in:
- Natural and synthetic fragrances: They contribute to the scents of fruits and flowers.
- Food flavorings: Many esters are responsible for the flavors we enjoy in various foods.
To fully appreciate esterification, it's essential to understand carboxylic acids. Here are some key points:
- Carboxylic Acid Formula: The general formula is RCOOH, where R is a hydrocarbon chain.
- Functional Group: The carboxylic acid functional group (-COOH) is what makes these compounds unique.
- Structure and Nomenclature: Carboxylic acids are named based on the length of their carbon chains and the presence of the carboxyl group.
Did you know that esters can also be formed through the reaction of carboxylic acids and sodium carbonate? This reaction is often used in laboratories to produce esters for various applications.
Understanding the process of esterification is crucial for:
- Organic synthesis: Creating new compounds for research and industry.
- Industrial chemistry: Producing materials like plastics and solvents.
In summary, esterification is not just a simple reaction; it's a gateway to a world of scents, flavors, and essential chemical processes. By mastering the concepts of carboxylic acids, you unlock the potential to explore a wide range of applications in chemistry! 🌟

| Reactants | Products | Catalyst | Characteristics |
|---|---|---|---|
| Carboxylic Acid (RCOOH) + Alcohol (R'OH) | Ester (RCOOR') + Water (H₂O) | Sulfuric Acid (H₂SO₄) | Reversible reaction; equilibrium can be shifted by removing water or using excess reactants; esters have pleasant odors. |
Acid chlorides, also known as acyl chlorides, are fascinating derivatives of carboxylic acids. They are formed through a specific reaction involving carboxylic acids and chlorinating agents like thionyl chloride (SOCl₂) or oxalyl chloride (COCl)₂. Let’s break down this process!
The general reaction can be summarized as follows:
RCOOH + SOCl₂ → RCOCl + SO₂ + HCl
Here’s what happens in this reaction:
This transformation is crucial because it converts a less reactive carboxylic acid into a more reactive acid chloride.
Acid chlorides play a vital role in organic synthesis due to their high reactivity. Here are some key points about their significance:
Versatile Intermediates: They can easily undergo further reactions to produce:
Reactivity: The increased reactivity of acid chlorides compared to their parent carboxylic acids makes them valuable in various chemical reactions.
Structure: Acid chlorides have a distinct structure that includes the carbonyl group (C=O) and a chlorine atom attached to the carbon. This structure is derived from the carboxylic acid functional group.
Nomenclature: The naming of acid chlorides follows specific rules, often derived from the parent carboxylic acid by replacing the -oic acid suffix with -oyl chloride.
To deepen your understanding of acid chlorides and their connection to carboxylic acids, consider exploring the following related topics:
By mastering the formation and reactivity of acid chlorides, you’ll enhance your grasp of organic chemistry and its applications. Keep experimenting and exploring these fascinating compounds! 🌟
| Reaction | Reagents | Products | Reactivity |
|---|---|---|---|
| Carboxylic Acid to Acid Chloride | RCOOH + SOCl₂ | RCOCl + SO₂ + HCl | More reactive than carboxylic acid |
| Acid Chloride to Ester | RCOCl + Alcohol | RCOOR' + HCl | Reactive intermediate for ester formation |
| Acid Chloride to Amide | RCOCl + Amine | RCONH₂ + HCl | Reactive intermediate for amide formation |
Amides play a crucial role in organic chemistry, particularly in the synthesis of pharmaceuticals and polymers. Let’s dive deeper into how these important compounds are formed!
Amides are organic compounds that contain a carbonyl group (C=O) linked to a nitrogen atom (N). They can be derived from two main sources:
- Carboxylic Acids: These are organic acids that contain a carboxyl functional group (-COOH).
- Amines: These are compounds derived from ammonia (NH₃) where one or more hydrogen atoms are replaced by alkyl or aryl groups.
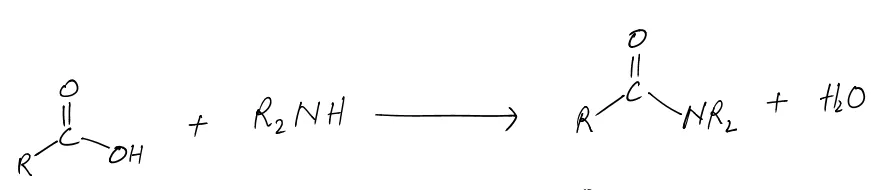
The formation of amides occurs through a condensation reaction, where a carboxylic acid reacts with an amine, resulting in the elimination of water (H₂O). The general reaction can be represented as:
RCOOH + R'NH₂ → RCONHR' + H₂O
Here’s a breakdown of the components:
- RCOOH: Represents the carboxylic acid.
- R'NH₂: Represents the amine.
- RCONHR': The resulting amide.
To enhance the reactivity of the carboxylic acid, it can be activated using various reagents. One common reagent is DCC (dicyclohexylcarbodiimide), which helps facilitate the reaction by forming an intermediate that is more reactive than the carboxylic acid alone.
Another efficient method to form amides is by using acid chlorides. Acid chlorides are more reactive than carboxylic acids, making the reaction with amines straightforward. The reaction proceeds as follows:
RCOCl + R'NH₂ → RCONHR' + HCl
This method is often preferred in laboratory settings due to its simplicity and higher yield.
Understanding amide formation is essential for several reasons:
- Pharmaceuticals: Many drugs are amides or contain amide bonds, making their synthesis vital for medicinal chemistry.
- Polymer Chemistry: Amides are key components in various polymers, contributing to their properties and applications.
To further your understanding of carboxylic acids and their role in amide formation, consider exploring the following topics:
- Carboxylic Acid Formula: Learn the specific formulas that define different carboxylic acids.
- Carboxylic Acid Functional Group: Understand the characteristics and importance of the -COOH group.
- Conversion of Carboxylic Acid to Alcohol: Discover how carboxylic acids can be transformed into alcohols through reduction processes.
By grasping the fundamentals of amide formation and the role of carboxylic acids, you can appreciate the intricate connections in organic chemistry and their practical applications! 🌈

| Reactants | Products | Reagents/Conditions | Notes |
|---|---|---|---|
| Carboxylic Acid (RCOOH) + Amine (R'NH₂) | Amide (RCONHR') + Water (H₂O) | Condensation reaction, possibly using DCC | Common method for amide formation. |
| Acid Chloride (RCOCl) + Amine (R'NH₂) | Amide (RCONHR') + HCl | More reactive than carboxylic acids, easier formation | Preferred method due to higher reactivity of acid chlorides. |
Preparing for exams can be a daunting task, but focusing on past paper questions is a game-changer! When it comes to carboxylic acids, understanding the types of questions that frequently appear can significantly boost your confidence and performance. Here’s a breakdown of the key question types you should master:
By focusing on these important types of questions related to carboxylic acids, you’ll be well on your way to mastering the topic! Remember to also review the carboxylic acid formula, functional group, and nomenclature as they are crucial for a comprehensive understanding. Happy studying! 🌟

| Question Type | Description | Key Concepts |
|---|---|---|
| Hydrolysis of Cyano Group | Understand the products formed when nitriles are hydrolyzed. | Nitriles, Hydrolysis, Carboxylic Acids |
| Reactions with Sodium Carbonate | Familiarize with how carboxylic acids react with sodium carbonate to produce carbon dioxide. | Carboxylic Acids, Sodium Carbonate, CO2 Production |
| Acid-Base Strength Comparisons | Compare the acidic strengths of carboxylic acids with other organic compounds. | Acidic Strength, Comparison, Organic Compounds |
| Esterification Mechanism | Explain the mechanism of ester formation from carboxylic acids and alcohols. | Esterification, Mechanism, Carboxylic Acids, Alcohols |
| Reactivity Trends | Understand the reactivity of various carboxylic acid derivatives. | Reactivity, Carboxylic Acid Derivatives |
In conclusion, carboxylic acids are a vital class of organic compounds characterized by the presence of the carboxyl functional group (-COOH). Their diverse properties, including solubility, boiling points, and reactivity, make them essential in various chemical reactions and applications. Understanding the nomenclature, classification, and preparation methods of carboxylic acids is crucial for mastering organic chemistry concepts, especially for students preparing for the MDCAT exam. By grasping the significance of the carboxylic acid formula and its structural characteristics, learners can better appreciate their role in biological systems and industrial processes. As you continue your studies, remember that the insights gained from this chapter will serve as a strong foundation for more advanced topics in chemistry.
A: The general formula for carboxylic acids is RCOOH, where R represents a hydrocarbon chain or hydrogen atom.
A: The functional group of carboxylic acids is the carboxyl group (-COOH), which consists of a carbonyl (C=O) and a hydroxyl (O-H) group.
A: Carboxylic acids are named using IUPAC nomenclature by identifying the longest carbon chain containing the carboxyl group and replacing the 'e' of the corresponding alkane with 'oic acid'.
A: When carboxylic acids react with sodium carbonate, they produce carbon dioxide (CO2), water, and a sodium salt of the carboxylic acid.
A: Yes, carboxylic acids can be converted to alcohols through a reduction reaction, typically using reducing agents like lithium aluminum hydride (LiAlH4).
A: The structure of a carboxylic acid consists of a carbon atom double-bonded to an oxygen atom (carbonyl) and single-bonded to a hydroxyl group (OH), forming the carboxyl group (-COOH).
A: The carboxylic acid functional group is significant in organic chemistry because it imparts acidic properties to compounds, allowing for various reactions such as esterification and amide formation.
A: Carboxylic acids can undergo polymerization to form polyesters, which are produced by the reaction of carboxylic acids with alcohols, resulting in the formation of ester linkages.
A: The carboxylic acid formula for fatty acids typically follows the general formula CnH2n+1COOH, where 'n' represents the number of carbon atoms in the hydrocarbon chain.
A: Cubane carboxylic acid refers to a carboxylic acid derivative of cubane, a hydrocarbon with a cubic structure, which can exhibit unique properties due to its geometric configuration.