Macromolecules are the fundamental building blocks of life, playing crucial roles in the structure and function of living organisms. Comprising large, complex molecules such as proteins, nucleic acids, carbohydrates, and lipids, macromolecules are essential for various biological processes. In this blog post, we will delve into the fascinating world of macromolecules, exploring their definitions, classifications, and examples. We will also discuss their significance in everyday life, including their presence in common substances like milk and their unique characteristics that set them apart from smaller molecules. Whether you are a student preparing for exams or simply curious about the molecular makeup of life, understanding macromolecules is vital. Join us as we unravel the intricacies of these remarkable compounds, from their definitions to their diverse functions in nature. By the end of this article, you will have a clearer grasp of what macromolecules are, their examples, and their importance in both chemistry and biology.
Macromolecules are large, complex molecules that are essential for life, primarily composed of smaller units called monomers. They include proteins, nucleic acids, carbohydrates, and lipids, each playing critical roles in biological processes. Proteins, for instance, are polymers of amino acids that perform various functions such as catalysis (enzymes), structural support, transport, and signaling. Nucleic acids, like DNA and RNA, are responsible for genetic information storage and transfer. Carbohydrates serve as energy sources and structural components, while lipids are crucial for membrane structure and energy storage. Understanding macromolecules is fundamental in biochemistry, as they are involved in virtually every cellular function. Their study encompasses various aspects, including their structure, function, and the biochemical pathways they participate in, making them a key focus in both academic and applied sciences.

Macromolecules are large, complex molecules that are fundamental to life. They play critical roles in biological processes and are essential for the structure and function of living organisms. The four main types of macromolecules include proteins, nucleic acids, carbohydrates, and lipids. Each type has unique structures and functions that contribute to the overall functioning of life.
Understanding macromolecules involves recognizing their key characteristics:
Size:
Diversity:
Biological Importance:
Let's dive into the main types of macromolecules and their functions:
Proteins 🥩
Nucleic Acids 🧬
Carbohydrates 🍞
Lipids 🥑
Understanding macromolecules is crucial for grasping the complexities of biological systems. From the proteins that catalyze reactions to the nucleic acids that carry genetic information, these molecules are the building blocks of life. Whether you're exploring macromolecules in milk or pondering if diamond is a macromolecule, the study of these large molecules reveals the intricate tapestry of life itself.

| Type | Composition | Function | Examples |
|---|---|---|---|
| Proteins | Amino acids | Catalysis, transport, structural support | Enzymes, Hemoglobin, Collagen |
| Nucleic Acids | Nucleotides | Storage and transmission of genetic information | DNA, RNA |
| Carbohydrates | Monosaccharides, Disaccharides, Polysaccharides | Energy storage, structural support | Glucose, Sucrose, Starch, Cellulose |
| Lipids | Fatty acids and glycerol | Energy storage, cell membrane structure | Fats, Oils, Phospholipids |
Understanding Proteins: The Building Blocks of Life 🧬
Proteins are one of the most important macromolecules in our bodies! They play a crucial role in the structure, function, and regulation of tissues and organs. Let’s dive deeper into what makes proteins so special!
Proteins are composed of smaller units called amino acids. These amino acids are linked together by peptide bonds, forming long chains known as polypeptides. Once formed, these chains fold into unique three-dimensional shapes, which are essential for their function.
Proteins are incredibly versatile and perform a variety of functions in the body. Here are some of the key roles they play:
Enzymatic Activity:
Structural Support:
Transport:
Defense:
Here are some common types of proteins and their functions:
Enzymes:
Structural Proteins:
Transport Proteins:
Hormones:
Proteins are classified as macromolecules because they are large, complex molecules made up of many smaller units (amino acids). They are essential for various biological functions and are one of the four main types of macromolecules, alongside carbohydrates, lipids, and nucleic acids.
Did you know that proteins can also be found in various foods? For example, macromolecules in milk include casein and whey proteins, which are great for muscle repair and growth!
Understanding proteins and their functions helps us appreciate the complexity of life and the importance of nutrition in our diets. So, the next time you think about macromolecules, remember the incredible role proteins play in keeping our bodies healthy and functioning!

| Function | Example |
|---|---|
| Enzymatic Activity | Pepsin, Trypsin |
| Structural Support | Collagen, Keratin |
| Transport | Hemoglobin, Myoglobin |
| Defense | Antibodies |
| Hormonal Regulation | Insulin, Glucagon |
Proteins are fascinating macromolecules that play crucial roles in biological systems. Their classification based on structure and function not only helps us understand their diverse roles but also highlights their importance in various biochemical processes. Let’s dive deeper into the classification of proteins! 🧬
Proteins can be categorized into three main types:
Simple Proteins:
Conjugated Proteins:
Derived Proteins:
Proteins can also be classified based on their functions in the body. Here are some key categories:
Enzymes:
Structural Proteins:
Transport Proteins:
Hormonal Proteins:
Understanding the classification of proteins not only enriches our knowledge of macromolecules but also sheds light on their vital roles in health and disease. Whether you're studying macromolecules in milk or exploring the question, is diamond a macromolecule?, the world of proteins is undeniably intriguing! 🌟
| Type of Protein | Description | Examples |
|---|---|---|
| Simple Proteins | Yield only amino acids upon hydrolysis. | Albumins, Globulins |
| Conjugated Proteins | Composed of simple proteins combined with non-protein components. | Glycoproteins, Lipoproteins |
| Derived Proteins | Formed from the hydrolysis of simple or conjugated proteins. | Peptides, Peptones |
The structure of proteins is vital for their function in biological systems. Proteins are complex macromolecules that play essential roles in various cellular processes. Understanding their structure helps us appreciate how they work and interact within living organisms.
Proteins have four distinct levels of structure, each contributing to their unique shape and functionality:
Primary Structure:
Secondary Structure:
Tertiary Structure:
Quaternary Structure:
Proteins are one of the major types of macromolecules, which also include carbohydrates, lipids, and nucleic acids. Understanding proteins as macromolecules helps us explore their roles in various biological processes.
By grasping the structure of proteins, we can better understand their role as essential macromolecules in life. 🌟

| Level of Structure | Description | Importance |
|---|---|---|
| Primary Structure | The sequence of amino acids in a polypeptide chain. | Determines the unique characteristics and function of the protein. |
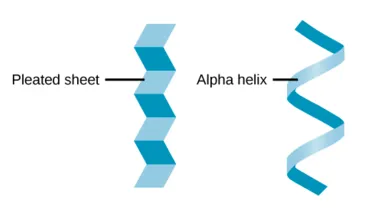
| Secondary Structure | Local folding into structures like alpha-helices and beta-pleated sheets, stabilized by hydrogen bonds. | Provides stability and defines the overall shape of the protein. |
| Tertiary Structure | The overall three-dimensional shape of a polypeptide, determined by interactions between R groups of amino acids. | Critical for the protein's function and interaction with other molecules. |
| Quaternary Structure | The arrangement of multiple polypeptide chains into a single functional protein (e.g., Hemoglobin). | Essential for the functionality of proteins composed of more than one chain. |
Denaturation of Proteins: Understanding the Process and Its Implications
Denaturation is a fascinating process that affects proteins, which are essential macromolecules in our bodies. When proteins undergo denaturation, they lose their native structure due to various external factors, resulting in a significant loss of function. Let's dive deeper into this intriguing phenomenon!
Denaturation can be triggered by several factors, including:
Heat 🌡️:
pH Changes ⚖️:
Chemicals 🧪:
The consequences of denaturation can be quite significant:
Understanding denaturation is crucial because proteins are one of the four main types of macromolecules essential for life. Here are some key points about macromolecules:
Understanding the denaturation of proteins not only highlights the importance of these macromolecules but also emphasizes the delicate balance required to maintain their structure and function.
| Cause | Description | Consequences |
|---|---|---|
| Heat | High temperatures disrupt hydrogen bonds and other interactions, causing proteins to unfold. | Loss of biological activity; may be irreversible. |
| pH Changes | Extreme changes in pH alter the charge of amino acids, affecting interactions that maintain structure. | Loss of biological activity; may be reversible if conditions return to normal. |
| Chemicals | Certain chemicals disrupt disulfide bonds or hydrogen bonds, leading to denaturation. | Loss of biological activity; may be irreversible. |
| Example | Coagulation of egg whites when heated. | Irreversible loss of function. |
Enzymes are specialized proteins that play a crucial role in speeding up chemical reactions in living organisms. Think of them as the superheroes of the biological world—working tirelessly to ensure that our bodies function smoothly without being consumed in the process.
Enzymes are essential for various physiological processes, including:
- Digestion: Breaking down food into nutrients.
- Metabolism: Converting food into energy.
- DNA Replication: Ensuring genetic information is accurately copied.
Understanding enzymes involves recognizing their unique characteristics:
Specificity:
Catalytic Efficiency:
Regulation:
Here are some key examples of enzymes and their functions:
Digestive Enzymes:
Metabolic Enzymes:
Enzymes are vital for the breakdown and synthesis of macromolecules, which are large, complex molecules essential for life. Understanding the relationship between enzymes and macromolecules can deepen your knowledge of biological processes.
Macromolecules are large molecules that are crucial for various biological functions. They include:
- Proteins: Made up of amino acids, essential for building and repairing tissues.
- Carbohydrates: Provide energy and structural support.
- Lipids: Important for cell membranes and energy storage.
- Nucleic Acids: DNA and RNA, which store and transmit genetic information.
By exploring enzymes and their interaction with macromolecules, we can appreciate the intricate dance of life at the molecular level! 🌍✨
| Characteristic | Description | Examples |
|---|---|---|
| Specificity | Each enzyme is specific to a particular substrate. | Amylase (starch), Protease (proteins), Lipase (fats) |
| Catalytic Efficiency | Enzymes increase reaction rates by lowering activation energy. | Hexokinase (glucose metabolism), Lactate Dehydrogenase (lactic acid production) |
| Regulation | Enzyme activity can be regulated by temperature, pH, inhibitors, and activators. | Temperature-sensitive enzymes, pH-dependent enzymes |
Enzymes are fascinating macromolecules that play a vital role in biological processes. They are primarily composed of proteins, and their intricate structure is essential for their function. Let's dive deeper into the components that make up these incredible molecules!
Apoenzyme:
Cofactor:
Holoenzyme:
By understanding the composition of enzymes, we can better grasp their significance in the world of macromolecules and their impact on biological systems!
| Component | Description | Importance |
|---|---|---|
| Apoenzyme | The protein component of an enzyme, which requires a cofactor for activity. | Determines the enzyme's specificity and function. |
| Cofactor | A non-protein component that assists in enzyme activity; can be a metal ion or an organic molecule. | Essential for the catalytic activity of the enzyme. |
| Holoenzyme | The complete active enzyme, consisting of the apoenzyme and its cofactor. | Represents the fully functional form of the enzyme, capable of catalyzing reactions. |
Enzymes are fascinating macromolecules that play a crucial role in biological processes by catalyzing reactions. However, their activity can be influenced by various factors. Understanding these factors is essential for manipulating enzymatic reactions in fields such as biotechnology, medicine, and food science. Let’s dive deeper into the key factors that affect enzyme activity! 🌟
Substrate Concentration
- As the concentration of substrate increases, the rate of reaction typically increases as well. This is because more substrate molecules are available for the enzyme to act upon.
- However, there comes a point where the enzyme becomes saturated—meaning all active sites are occupied. Beyond this point, increasing substrate concentration will not further increase the reaction rate. 🚀
Enzyme Concentration
- Increasing the concentration of enzymes can lead to a higher reaction rate, provided there is enough substrate available.
- More enzymes mean more active sites for the substrate to bind to, which can significantly enhance the reaction speed. Just remember, if the substrate is limited, adding more enzymes won’t make a difference! ⚗️
Temperature
- Each enzyme has an optimal temperature range where it functions best.
- Too high temperatures can lead to denaturation, where the enzyme loses its shape and, consequently, its function.
- Conversely, too low temperatures can slow down molecular movement, reducing the reaction rate. Finding that sweet spot is key! 🌡️
pH Levels
- Similar to temperature, each enzyme has an optimal pH level. Deviations from this range can affect the enzyme's structure and activity.
- For example, pepsin, an enzyme in the stomach, works best in acidic conditions, while others may require neutral or alkaline environments. 🌈
Inhibitors
- Inhibitors are molecules that decrease enzyme activity. They can bind to the enzyme or the substrate, preventing the reaction from occurring.
- There are two main types of inhibitors:
Activators
- Activators are molecules that increase enzyme activity. They enhance the enzyme's ability to bind to the substrate, thus speeding up the reaction.
- Some activators can change the enzyme's shape, making it more effective at catalyzing reactions. 🌟
Understanding these factors is essential for anyone working with enzymes, especially in fields related to macromolecules. By manipulating conditions such as substrate concentration, temperature, and pH, we can optimize enzyme activity for various applications, from industrial processes to medical treatments. So next time you think about enzymes, remember these key factors that can make or break their activity! 💡
| Factor | Description | Impact on Enzyme Activity |
|---|---|---|
| Substrate Concentration | The amount of substrate available for the enzyme to act upon. | Increases reaction rate until saturation is reached. |
| Enzyme Concentration | The amount of enzyme present in the reaction. | Higher concentrations increase reaction rates if substrate is sufficient. |
| Temperature | The degree of heat present in the environment. | Each enzyme has an optimal temperature; extreme heat can denature enzymes. |
| pH | The acidity or basicity of the environment. | Each enzyme has an optimal pH; deviations can reduce activity. |
| Inhibitors | Molecules that bind to the enzyme or substrate and reduce activity. | Decrease the rate of reaction. |
| Activators | Molecules that enhance enzyme activity. | Increase the rate of reaction by improving substrate binding. |
Importance of Enzymes 🌟
Enzymes are vital for life, acting as catalysts that facilitate numerous biochemical reactions essential for sustaining living organisms. Their significance stretches across various biological processes, industries, and medical applications. Let’s dive deeper into the importance of enzymes and their relationship with macromolecules.
Enzymes are crucial players in many biological processes. Here are some key areas where they shine:
Metabolism:
Digestion:
DNA Replication and Repair:
Enzymes are not just important in biological systems; they also have significant industrial applications:
Biotechnology:
Food Industry:
The medical field also benefits greatly from enzymes:
Diagnostics:
Therapeutics:
In summary, enzymes are indispensable for life, playing critical roles in metabolism, digestion, and genetic processes. Their applications in biotechnology, food production, and medicine highlight their versatility and importance. Understanding enzymes and their relationship with macromolecules can provide deeper insights into both biological and industrial processes.
| Category | Importance | Examples |
|---|---|---|
| Biological Importance | Metabolism | Enzymes regulate metabolic pathways, allowing organisms to convert food into energy. |
| Digestion | Digestive enzymes break down complex food molecules into absorbable units. | |
| DNA Replication and Repair | Enzymes play crucial roles in the processes of DNA synthesis and repair, ensuring genetic integrity. | |
| Industrial Applications | Biotechnology | Enzymes are used in genetic engineering, fermentation, and bioremediation. |
| Food Industry | Enzymes are employed in food processing, such as brewing, baking, and cheese production. | |
| Medical Applications | Diagnostics | Enzymes are used in various diagnostic tests to measure enzyme levels in blood, indicating health conditions. |
| Therapeutics | Enzyme replacement therapy is used to treat certain genetic disorders. |
Understanding macromolecules is essential for grasping the fundamentals of biochemistry and biology. These large, complex molecules play a vital role in the structure and function of living organisms. Let's dive into some key questions that can help reinforce your knowledge about macromolecules! 🌟
Proteins are incredibly versatile and serve multiple functions, including:
- Catalysis: Acting as enzymes to speed up chemical reactions.
- Structural Support: Providing shape and strength to cells and tissues.
- Transport: Carrying molecules across cell membranes or throughout the body.
- Signaling: Facilitating communication between cells.
Proteins can be classified in several ways:
1. By Structure:
- Simple Proteins: Composed only of amino acids.
- Conjugated Proteins: Contain additional non-protein components.
- Derived Proteins: Fragments of proteins that have been broken down.
2. By Function:
- Enzymes: Catalyze biochemical reactions.
- Structural Proteins: Provide support and shape.
- Transport Proteins: Move substances across membranes.
- Hormones: Act as signaling molecules.
Enzyme activity can be influenced by several factors:
- Substrate Concentration: More substrate can increase reaction rates until saturation.
- Enzyme Concentration: Higher enzyme levels can enhance reaction speed.
- Temperature: Each enzyme has an optimal temperature; too high or too low can denature them.
- pH Levels: Enzymes work best at specific pH levels; deviations can affect activity.
- Inhibitors: Molecules that decrease enzyme activity.
- Activators: Molecules that increase enzyme activity.
Enzymes are crucial for numerous processes, including:
- Metabolic Processes: They facilitate the biochemical reactions necessary for life.
- Digestion: Breaking down food into absorbable units.
- DNA Replication: Ensuring accurate copying of genetic material.
- Industrial and Medical Applications: Used in pharmaceuticals, food processing, and biotechnology.
By understanding these key aspects of macromolecules, you can appreciate their significance in biology and chemistry. Whether you're exploring macromolecule examples or diving into the differences between micromolecules and macromolecules, the knowledge of these large molecules is foundational to the life sciences! 🌍
| Question | Answer |
|---|---|
| What are macromolecules? | Macromolecules are large, complex molecules essential for life, including proteins, nucleic acids, carbohydrates, and lipids. |
| What is the primary function of proteins? | Proteins serve various functions, including catalysis (enzymes), structural support, transport, and signaling. |
| How are proteins classified? | Proteins can be classified based on their structure (simple, conjugated, derived) and function (enzymes, structural proteins, transport proteins, hormones). |
| What factors affect enzyme activity? | Factors include substrate concentration, enzyme concentration, temperature, pH, inhibitors, and activators. |
| Why are enzymes important in biological systems? | Enzymes are crucial for metabolic processes, digestion, DNA replication, and various industrial and medical applications. |
In conclusion, understanding macromolecules is essential for grasping the fundamental principles of biochemistry and biology. These large, complex molecules, including proteins, carbohydrates, lipids, and nucleic acids, play critical roles in the structure and function of living organisms. From the structural support provided by proteins to the energy storage capabilities of carbohydrates, macromolecules are integral to life processes. As we have explored various examples of macromolecules, their classifications, and functions, it becomes clear that they are not only vital for cellular activities but also for overall organismal health. By appreciating the significance of macromolecules, students can better prepare for their MDCAT exams and develop a deeper understanding of biological systems.
A: Macromolecules are large, complex molecules that are essential for life. They include proteins, nucleic acids, carbohydrates, and lipids. These molecules are made up of smaller units called monomers, which join together to form polymers.
A: Examples of macromolecules include proteins (like enzymes and antibodies), nucleic acids (DNA and RNA), carbohydrates (starch and cellulose), and lipids (fats and oils).
A: A macromolecule is a large molecule composed of thousands of atoms, typically formed by the polymerization of smaller subunits known as monomers. They play crucial roles in biological processes.
A: Micromolecules are small molecules, often consisting of a few atoms, such as water, glucose, and amino acids. In contrast, macromolecules are larger and more complex, consisting of many atoms and often formed by the combination of micromolecules.
A: Yes, milk contains several macromolecules, including proteins (like casein and whey), carbohydrates (lactose), and fats (milk fat). These macromolecules are essential for nutrition.
A: Yes, diamond can be considered a macromolecule because it consists of a large number of carbon atoms bonded together in a crystalline structure, forming a giant covalent network.
A: No, sodium is not a macromolecule. It is a chemical element and a simple ion (Na+) that does not form large, complex structures like macromolecules do.
A: Macromolecules are significant in chemistry as they are fundamental to the structure and function of living organisms. They participate in various biochemical processes, including metabolism, genetic information storage, and cellular structure.